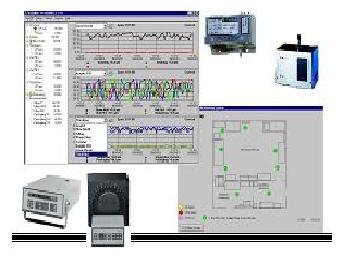
CGMP Pharm Particle Monitor System
Model No.︰2400
Brand Name︰HACH ULTRA(PSI)/MetOne2400/2100
Country of Origin︰United States
Unit Price︰-
Minimum Order︰-
Product Description
“A remote counting
system is generally less
invasive than portable
counting units”
- FDA Guidance for Industry
In addition to significantly improved data, there is a
secondary financial benefit to continuous monitoring key
areas. ISO 14644-2 permits the interval between formal,
and expensive, certification of areas to be extended beyond
the stated six months for ISO Class 5 areas and 12 months
for ISO Class 6, 7, 8, or 9 areas. Although the longest period
that regulators might be comfortable with a user not having
certified a key area would be about 24 months, this still
would mean only one certification over two years. For a grade
A, ISO Class 5 area, four certifications in 24 months is the
specified requirement.
Continuous or nearly continuous airborne particle
monitoring can be an effective aid to maintaining the stability
of a process and the quality of a product. Choosing the key
points where the product is exposed and vulnerable as sampling
points will provide solid information to help ensure the quality
of a product.
specifications︰
CGMP Pharm Particle Monitoring System
• 0.1-1.0£gm; 0.3-10 or 25£gm ·L²É¤l¶q´ú½d³ò¡C
Advantages︰
• ¿W¥ßparticle sensors ³sÄò¶q´ú¤è¦¡¡C
• ³æ¾÷¦hÂI(32 ports)³sÄò½ü¬y¶q´ú¤è¦¡¡C
• ¾A¥Î©óµL¹Ð«Çclass 1 to 100k, mini-environment ¡C
• ¥iµ²¦X·ÅÀã«×p, ·³tp, ®tÀ£p, ESD sensorµ¥ Ãþ¤ñ¦¡°T¸¹·P´ú»ö¾¹¡C
Product Image